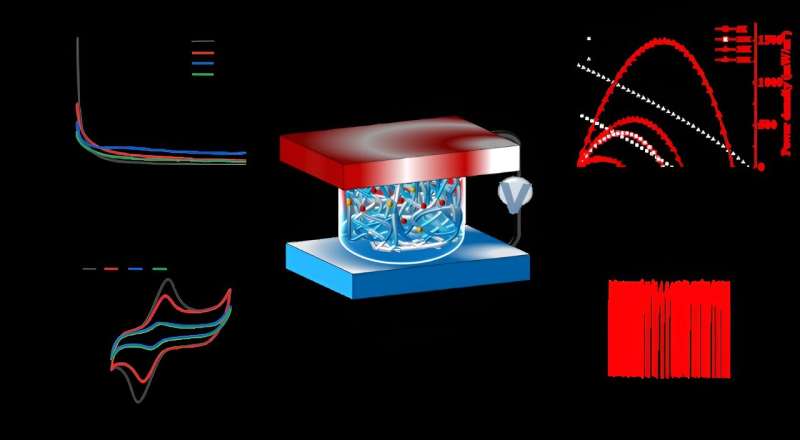
The electrode sheet of a thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction to improve the energy density and power density of the ionic thermoelectric generator.
New research on this topic appears in Energy Material Advances.
Prof. Zeng Wei of the Institute of Chemical Engineering, Guangdong Academy of Sciences, said that at the beginning, the research team mainly carried out study based on the thermal diffusion effect and published a series of research results. In spite of this, their results never realized the expected effect, and the prospect of practical application was not optimistic.
Later, they tried to make a further enhancement on the basis of the thermal current effect; that is, to incorporate the redox reaction of the electrode. The reason for this is that the thermal current effect is redox in the electrolyte, so the gain and loss of electrons mainly occur in the solution, and the electrons in the electrolyte to migrate to the electrode are not only more difficult, but also need to travel a distance, which will lead to both a lower conversion efficiency and ineffective loss of electrons.
If redox can be achieved directly at the electrodes, that is, if ions are allowed to reach the electrodes and then undergo redox reactions in a thermally induced manner, rather than being driven by an electric current, the distance traveled by the electrons can be very well reduced, resulting in high thermoelectric conversion efficiencies and a significant increase in the time that the thermoelectric device can supply power to the outside world.
“In this work the instantaneous power density reached 3.7 mW/m2K2. In addition, the output energy density was 194 J/m2 for 2 hours at a temperature gradient of 10 K, and the Carnot relative efficiency was as high as 0.12% at a hot-side temperature (TH) of 30°C and a cold-side temperature (TC) of 20°C,” Zeng said.
Therefore, in terms of applications, the device is already capable of continuously powering electronic devices such as wearable electronics and sensors. In addition, the team would like to further broaden the applications, such as using the device for solar photo-thermal power systems and heat recovery outside building walls; specifically, the temperature at which sunlight hits a solar panel is usually between 60 and 80 degrees Celsius, which is a few tens of degrees Celsius difference from the real ambient temperature.
If the currently developed thermoelectric device is attached to the back of the solar panel, it can further convert the wasted heat energy into electricity, thus increasing the efficiency of solar energy output. By using the devices for heat recovery outside the building walls, the purpose of powering the building itself can be realized.
Talking about the follow-up plan of this research, Zeng said that at present, the main use of polyaniline to modify the electrode, its redox characteristics and capacity is relatively limited. Therefore, the next step is to find more materials that correspond to the thermal potential under study to further increase the density of the redox electrodes and the energy output to the outside world.
At the same time, the team also plans to improve the specific capacitance of the electrodes and increase the specific surface area to better increase the capacity ratio of the electrodes. In addition, they will continue to optimize the structural design of the hydrogel itself and broaden the choice of materials.
Other contributors include Xia Yang, Dongyu Zhu, Guangdong University of Technology; Fei Wang, Chen Wu and Jianchao Jia, Institute of Chemical Engineering, Guangdong Academy of Sciences; and Jin Liu, Department of Mechanical and Aerospace Engineering, Hong Kong University of Science and Technology.