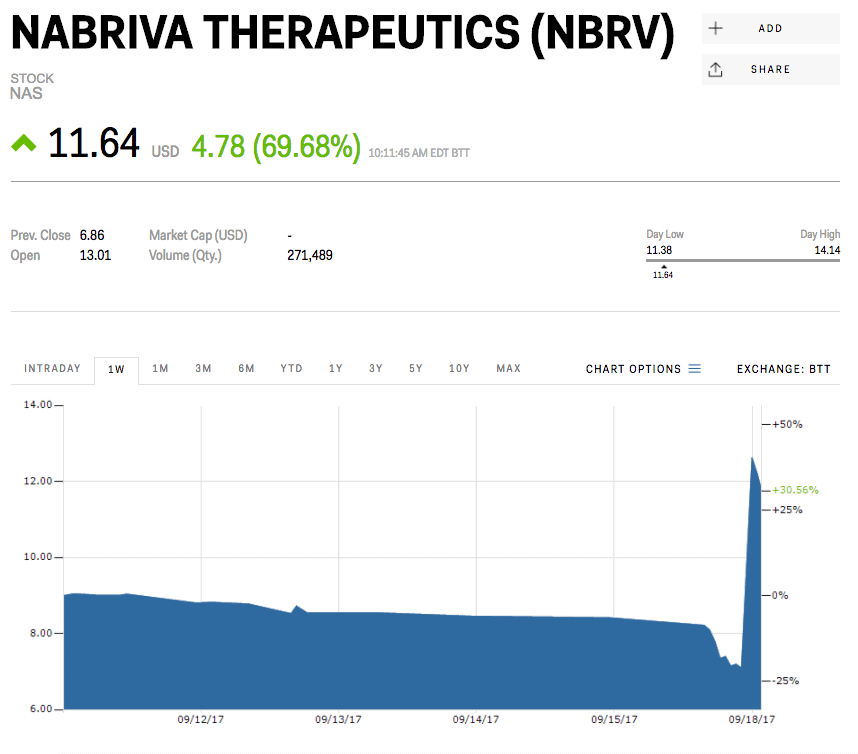
 Shares of Nabriva Therapeutics more than doubled in premarket trading on Monday after the company said its experimental antibiotic for community-acquired bacterial pneumonia met the main goal of a late-stage study.
Shares of Nabriva Therapeutics more than doubled in premarket trading on Monday after the company said its experimental antibiotic for community-acquired bacterial pneumonia met the main goal of a late-stage study.
The clinical trial — the first of two late-stage studies — showed that the company’s drug, lefamulin, was as effective as the commonly used antibiotic moxifloxacin, which was the main goal of the trial.
Community-acquired bacterial pneumonia is one of the most common infectious diseases and the leading cause of infectious death in the United States.
The company’s shares jumped to as much as $13.91 in premarket trading from their Friday close of $6.86.
Why new antibiotics are critical
Antibiotic-resistant diseases are expected to kill 10 million people annually by 2050 . And it hasn’t been easy to get new drugs to stay ahead of the problem, especially for common infections like pneumonia. Many major pharmaceutical companies have stopped developing new antibiotics, and the drugs that are still in development have faced numerous stumbling blocks toward approval .
Government organizations such as the Centers for Disease Control and Prevention have been warning about the rise of antibiotic resistance, saying we’ll soon be in a “post-antibiotic era.”
Should the second of Nabriva’s two late-stage trials pan out and lefamulin gets approved, it could give doctors a new line of treatment to stay ahead of that age.























