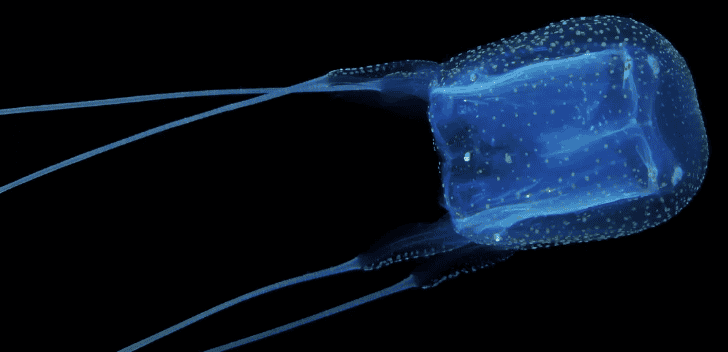
The transparent box jellyfish is one of the world’s most venomous animals, patrolling tropical waters with a trail of 3 meter long, streamer-like tentacles ready to deliver a painful, and potentially fatal, venom.
Of the numerous species of box jellyfish Chironex fleckeri is regarded as one of the most deadly. Its explosive sting can cause cardiac arrest in humans but scientists have not been able to pin down exactly how the jellyfish’s venom works. Now, a team of researchers have dissected the jellyfish with a powerful gene-editing tool, known as CRISPR, stumbling upon a potential new way to block its venom.
The research, published in Nature Communications on April 30, tested box jellyfish venom in human cells grown in the lab. Using CRISPR, which can make precise DNA edits, the team were able to create human cells with specific genes turned off. If they then applied the jellyfish venom to the cells, they could see which cells lived or died and determine which genes were important for keeping the cell alive.
“What we do is grow up millions of human cells, then we use CRISPR to knock out every gene in the human genome,” says Greg Neely, a functional geneticist at the University of Sydney, who led the research.
Think of the experiment like a library where every book is exactly the same. They’re all “Harry Potter and the Philosopher’s Stone”, OK? The team pulled out one copy and ripped out a page, then pulled out another copy and ripped out a different page. They did this hundreds of times. Now the library is full of different versions of “Harry Potter and the Philosopher’s Stone”, each missing a different page from the story.
Sometimes, you’re going to throw out a page that is crucial to the story. For instance, you might throw out the page with the famous “You’re a wizard, Harry” and now the story just isn’t going to work.
The team were hunting for the equivalent of “You’re a wizard, Harry” in the human genome — a key gene that would render the venom useless.
They were able to nail down which human genes caused the box jellyfish venom to be so deadly and which pathway the venom used to destroy cells. They found that four particular genes from a cholesterol regulation pathway were important in this process and so they honed in on them.
Importantly, we already have drugs for these pathways — and so the team decided to see how those drugs would work in preventing the venom from acting on human cells. The drugs — which team leader Neely called a “cholesterol sponge” — worked and, importantly, blocked the venom even 15 minutes after toxins were delivered.
“The drug is known to work by pulling cholesterol out of the cell membrane, so we think the jellyfish venom needs membrane cholesterol to exert its effect,” says Neely. “By shutting down this pathway for a short period of time, we can shut down the venom death pathway.”
That’s a handy fact, because when you’re stung by a box jellyfish, you’re likely in the water and not carrying around an antivenom.
The team will look to partner up with first responders and government to push the antidote forward as a potential treatment for human use.
Expect a major asteroid strike in your lifetime, NASA head says: Visions of space rocks slamming into Earth aren’t just for dinosaurs and Hollywood movies.
Drone delivers life-saving kidney for successful implant: Surgeons receive a donated organ via an unmanned aerial system and use it on a real patient.
























