Over the past decades, engineers and materials scientists have been working hard to develop battery technologies that exhibit increasingly better performances. Their efforts are aimed at supporting the needs of the electronics industry, increasing the battery life of countless rechargeable devices, electric vehicles, and robotic systems.
Nickel (Ni)-rich layered materials have been often employed as cathodes in battery cells, due to their advantageous properties that can increase the cells’ energy density. Nonetheless, the capacity of Ni-rich cathodes can rapidly deteriorate, due to the materials’ reactivity that causes undesirable side chemical reactions.
Researchers at Hanyang University in Seoul recently introduced a new strategy that could help to enhance the performance of these cathodes, allowing them to retain their capacity for longer periods of time. This strategy, introduced in a paper published in Nature Energy, involves a simple washing process that helps to remove residual lithium compounds on the cathodes, while also coating them to reduce unwarranted reactions.
“The instability of the Ni-rich layered cathode materials in lithium-ion batteries is attributed to their labile surface reactivity,” Hoon-Hee Ryu, Hyung-Woo Lim and their colleagues wrote in their paper. “This reactivity induces the formation of residual lithium impurities on the cathode surface and severe side reactions with the electrolyte. We propose a washing process using Co-dissolved water for simultaneously removing residual lithium and forming a protective coating on Ni-rich layered cathodes.”
The key objective of the recent work by this team of researchers was to identify an effective method to overcome the limitations of Ni-rich cathodes, which could in turn help to increase the duration of batteries in time. Their proposed strategy entails washing the cathodes with a cobalt (Co)-dissolved aqueous solution.
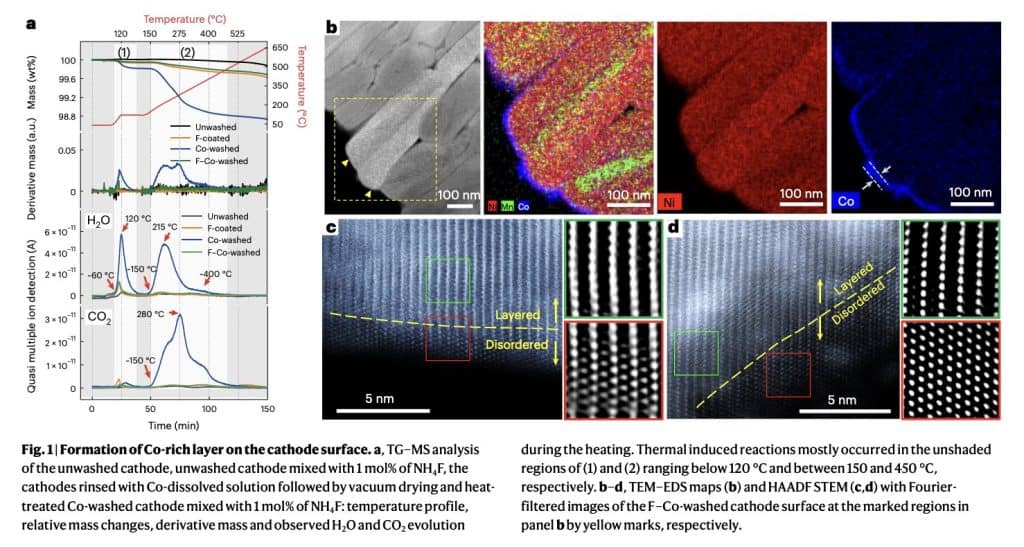
This washing process elicits a reaction with residual lithium on the outer surface of the cathodes, leading to the formation of a thin, uniform and Co-rich protective layer. The formed layer eliminates direct contact between the cathode and the electrolyte inside the battery cell, thus preventing further side reactions and the consequent rapid degradation of the cathode over time.
“The washing induces the reconstruction of the near-surface structure through reactions with the residual lithium compounds, thereby preventing direct contact between the electrolyte and the Ni-rich surface,” Ryu, Lim and their colleagues explained. “An additional fluorine coating on the washed cathode impedes the decomposition of salts, preventing the by-products from triggering autocatalytic side reactions at the electrolyte–cathode interface and thereby suppressing gas generation during cycling.”
The washing and coating strategy introduced by Ryu, Lim and their collaborators was tested in a series of initial experiments. Results showed that it successfully extended the life cycle of Ni-rich cathodes, without compromising their energy density and safety.
In the future, this recent work could be used to develop safer and more durable batteries for electric vehicles and other large-scale electronics. In addition, other research teams could draw inspiration from its findings to devise other promising methods to prevent the rapid capacity deterioration commonly reported in Ni-rich cathodes.























